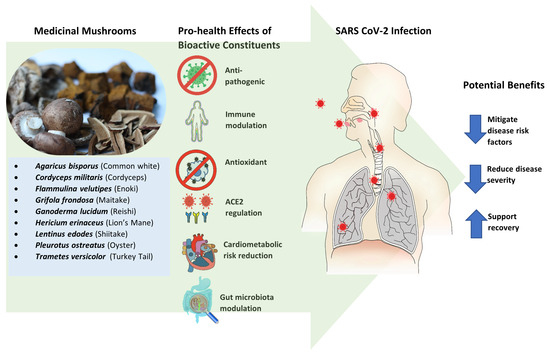
The following synopsis examines the various pro-health effects and activities of specific macro-fungi elucidated in research that may have applications to the pathological sequelae and outcomes associated with COVID-19. The effects and potential benefits of MMs are illustrated in .
6.1. Anti-Pathogenic
The basic viral cycle, also associated with SARS CoV-2, involves attachment, penetration, uncoating, replication, assembly, and release [
40]. Mushroom extracts and bioactive compounds impede viral entry into host cells and multiplication, inhibit virus adsorption, replication, nucleic acid synthesis, and disrupt other pathogens [
3,
6,
14]. It is known that proteolytic enzymes facilitate the cleavage of S glycoprotein, which is a critical step in SARS CoV-2 viral attachment [
12]. Since protease inhibitors have been isolated from
G. lucidum,
C. militaris, and
A. bisporus [
14], MMs may have therapeutic utility.
Cordycepin isolated from
C. militaris exerted an antiviral effect through a protein kinase inhibitory mechanism and an inhibitory role towards ribonucleic acid (RNA) synthesis and Epstein–Barr virus (EBV) replication [
41]. Ganoderma compounds isolated from
G. lucidum effectively inhibited human immunodeficiency virus (HIV)-1 and HIV-1 protease [
42]. Additionally, various triterpenoids (isolated from
G. lucidum and other Ganoderma species) were active against HIV-1, influenza type A, and herpes simplex type 1 [
6]. In vitro and in vivo studies on a range of common viral agents from
Agaricus spp. including
Agaricus blazei Murril (AbM),
H. erinaceus, and
G. frondosa demonstrated antiviral properties [
3].
MMs are also purported to have anti-bacterial functions [
1]. To confirm this potential, Hearst et al. [
43] conducted an in vitro microbiological assessment using aqueous extracts of
L. edodes and
P. ostreatus. The aqueous extract of
L. edodes demonstrated potent activity when tested in culture against 29 bacterial isolates (Gram-positive and Gram-negative) and 10 fungal/yeast agents. Here, 85% of the bacterial and 50% of the fungal organisms were inhibited by the
L. edodes extract. The results compared favourably against Ciproflaxin, which is a broad-spectrum antibiotic that was deployed as the control. In contrast,
P. ostreatus aqueous extract showed minimal activity on the same range of pathogens, with only three out of 39 samples inhibited, while none of the yeast and mould species was affected. Additionally, a purified source of lentinan, a specific class of
𝛽�-glucan, reduced populations of multiple antibiotic-resistant clinical isolate
Klebsiella pneumoniae in an in vivo lung infection model and showed potential for treating sepsis-induced lung injury and boosting type 1 interferon response to RNA viruses such as influenza and coronavirus [
17].
6.2. Immune Modulation
A response elicited from
L. edodes named “the lentinan antiviral effect” has been attributed to innate immune responses and specific immunity regulation. Acting as a BRM, lentinan can promote T helper cell (Th) type 1 response and improve Th1/Th2 balance. It may also activate inflammasomes, enhance immune cells, activate the complement system, and promote cytotoxicity and phagocytosis [
2,
44]. An in-house hot water extract of
L. edodes was compared to a commercially sourced lentinan extract (Carbosynth–Lentinan (CL)) to investigate if isolates could alleviate the immune cascade in conditions experienced by COVID-19 patients, such as ARDS.
𝛽�-glucans from
L. edodes reduced IL-1
𝛽� and IL-6 in lung injury and activated macrophages in vitro [
17].
𝛽�-glucans were also used to investigate oxidative stress alleviation in H
2O
2-treated THP-1 cells. Viability, apoptosis and necrosis were assessed. CL extract attenuated oxidative stress-induced early apoptosis, and the in-house lentinan extract attenuated late apoptosis [
17].
Lectin derived from
P. ostreatus has been studied as a hepatitis B virus DNA vaccine adjuvant and demonstrated effectiveness in enhancing surface protein antibodies [
45]. Studies utilising pleuran (insoluble
𝛽�-glucans derived from
P. ostreatus) administered in oral liquid syrup form have suggested numerous positive immunomodulatory effects in recurrent upper and lower respiratory tract infection (RRTI). Demonstrated effects of pleuran, particularly in studies with children, include reduced incidences of RRTI, otitis media, tonsillopharyngitis, bronchitis, laryngitis, and other flu and cold-like symptoms, plus fewer days off school [
46,
47,
48].
AbM extract is another rich source of BRMs. Via the actions of, for example, proteoglycans,
𝛽�-glucans, and ergosterol, anti-inflammatory, anti-pathogenic, and immunomodulatory cytokine effects were stimulated, vaccine efficacy was improved, and cytotoxic effects were induced [
49,
50,
51,
52]. Andosan™, a product primarily manufactured from AbM extract, combined with
H. erinaceus and
G. frondosa, has been investigated in clinical studies [
3]. Independently, these three mushrooms have demonstrated efficacy for their immunomodulatory, anti-infective, antitumour, and anti-inflammatory effects with reduced pro-inflammatory cytokines and oxidative stress, and beneficial gut microbiota responses [
52].
H. erinaceus contains aromatic compounds such as hericerins and erinacines that appear to function as a nerve growth factor as well as the beneficial immunomodulating and antitumour properties derived from the glycoproteins and polysaccharides [
52]. Further highlighting this, polysaccharides extracted from liquid-cultured mycelia and fruiting bodies of
G. frondosa demonstrated antioxidant, antitumour, anti-inflammatory, hepatoprotection, and immunostimulatory activity [
53]. Grifolan, a
𝛽�-glucan isolated from
G. frondosa, showed enhanced cellular immunity and modulation activities evidenced by increasing IL-2 and IL-10 production and augmentation of IL-6, IL-1, and TNF-
𝛼� expression [
54].
Water extract of four different MMs, including
G. lucidum, caused NK cell-induced cytotoxicity against cancer cells, but an ethanol extract did the opposite by reducing intracellular pathway activation [
55]. Various triterpene acids and sterols isolated from
G. lucidum fruiting bodies revealed antitumour and anti-inflammatory effects as demonstrated via induction of EBV early antigen by 12-O-tetradecanoylphorbol-13-acetate [
56].
T. versicolor has a long traditional history of use to promote health, strength, and longevity. More recently, numerous studies, including clinical trials, suggest properties and effects that include antimicrobial, antiviral, antitumour, anti-inflammatory, antioxidant, hepatoprotective, bone protective, and notably immunopotentiation [
57,
58]. Two bioactive mycelia extracts of protein bound polysaccharides from
T. versicolor, namely polysaccharopeptide (PSP) and polysaccharide krestin (PSK), are currently utilised medicinally in some countries as integrated cancer therapy and adjuncts for chemotherapy and radiotherapy [
2,
57,
59]. From a range of randomised and non-randomised controlled trials, both PSK and PSP promoted positive impacts on anticancer effects [
60]. Deemed resulting from the immunomodulation and potentiation of immune surveillance, PSK and PSP positively affect immune parameters, haematological function, performance status, quality of life, body weight, fatigue, pain, nausea, anorexia, and median survival [
59,
60,
61]. Additionally, antitumour and antimetastatic effects were noted through direct tumour-inhibiting experiments in vivo [
60]. Of interest in the context of COVID-19 application is the mechanisms of
T. versicolor. This appears to be through the inducement of predominantly pro-inflammatory cytokines: not only those associated with TNF-
𝛼� and NK cells but also pleiotropic cytokines such as IL-1
𝛼� and 1
𝛽� and IL-6, plus prostaglandin E2, histamine, activation of complement-3, and T cell proliferation [
57,
59,
61]. While this may be desired to improve cancer outcomes, such as enhancing the immunosuppressive status, a cautionary approach in applying
T. versicolor due to the hyperinflammatory response associated with COVID-19 progression should be taken. However, perhaps, there may be a place for consideration in the context of long COVID or playing a role as a vaccine adjuvant.
6.3. Antioxidant
The antioxidant/ROS system plays a significant role in pathogenic protection, regulation, and homeostasis in the human body. For example, the increased activity of ROS is a key feature in the pathogenesis and progression of many disease states such as atherosclerosis, arterial thrombosis, hyperlipidaemia, hypertension, cancer, obesity, insulin resistance, diabetes mellitus, hepatic and renal conditions, amongst many others [
62]. These disease states are representative comorbidities associated with SARS CoV-2 and COVID-19 sequalae and experience. The antioxidant capacity of MMs has been demonstrated in various studies through radical scavenging, lipid peroxidation inhibition, and increasing antioxidant enzyme activities [
30,
54,
63,
64]. Bioactive compounds such as phenolics, indoles, flavonoids, glycosides, polysaccharides, tocopherols, glutathione and ergothioneine, ascorbic acid, carotenoids, vitamin D, copper, manganese, zinc, and selenium in MMs all participate in reducing oxidative stress [
62,
65]. Ergothioneine deserves special mention, as it has a vast array of unique cytoprotective properties pertinent to COVID-19 pathologies, including scavenging reactive oxygen and nitrogen species. It is able to modulate inflammation, inhibit the expression of vascular adhesion proteins, and protect against respiratory burst, amongst many other antioxidant activities [
63]. Notable amounts of bioavailable ergothioneine were demonstrated in the fruiting bodies of
A. bisporus [
66],
L. edodes,
P. ostreatus, and mycelia of
C. militaris (strain cm5),
H. erinaceus, and
P. eryngii [
67,
68].
Liquid–liquid partitioned fractions of
H. erinaceus were evaluated for their anti-atherosclerotic potential through evaluation of in vitro inhibitory effect on low-density lipoprotein (LDL) oxidation and 3-hydroxy-2methylglutaryl coenzyme A (HMG-CoA) reductase activity [
69]. Several bioactive compounds with antioxidant activity were isolated, in particular ergosterol. Hexane solvent fraction demonstrated the most potent inhibiting oxidisation of LDL and HMG-CoA reductase activity. This indicates a possible role in preventing oxidative stress-mediated vascular disease processes [
69].
Radical scavenging properties associated with catalase activity, glutathione reductase, and glutathione peroxidase activities were demonstrated in varying degrees from methanol and water extracts isolated from the gills, stipe, and caps of two wild strains and one cultivated strain of
A. bisporus [
70]. Fourteen selected culinary MMs were evaluated for in vitro antioxidant and ACE inhibitory activities [
30]. The mushrooms were extracted by boiling water for 30 min. The total phenolic content was determined with
G. lucidum demonstrating the highest phenolic content and the most potent ACE inhibitor. Antioxidant capacity was carried out via measuring the free radical scavenging effect,
𝛽�-carotene, lipid peroxidation, reducing power ability, cupric-ion-reducing antioxidant capacity, and ACE inhibition. An antioxidant index was determined based on the average percentage relative to quercetin.
G. lucidum and
H. erinaceus were shown to be relatively high compared to the other mushrooms [
30].
6.4. ACE2 Regulation
The deleterious effects of COVID-19, such as those associated with cardiometabolic and other hallmark disorders, demonstrate dysregulation of the homeostatic function within the RAAS [
7,
13,
71,
72]. RAAS maintains dynamic control of vascular function. ACE2 is an integral membrane protein present in the lungs, liver, heart, kidney, and endothelium. ACE2 dysregulation appears to strongly impact the RAAS, manifesting effects involving hyperinflammation and oxidative stress. MMs have been investigated for ACE inhibitory, antiplatelet, anti-inflammatory, and antioxidant activity [
30,
73,
74].
In MMs, bioactive compounds such as triterpenes, sterols, phenolic compounds, and polysaccharide fractions possess metabolic-modulating capabilities [
54]. These include blood pressure, glycaemia, cholesterol, triglyceride, and weight-lowering activities. The ACE inhibitory activity of several mushroom species was assessed via hot water and alcohol extracts [
30].
G. lucidum, particularly as a hot water extract, and
Pleurotus spp. demonstrated potent ACE inhibitory activity, which is assumed to be due to the phenolic content and antioxidant capacity. However, variations existed between species and depended on the extraction method [
30]. In vitro digestion of
P. ostreatus identified several peptides known to be ACE inhibitors [
75]. A randomised, double-blind prevention trial is underway in the Democratic Republic of the Congo involving Tomeka
®, a herbal mixture containing
A. bisporus and other food-based nutrients such as soy, which is regarded for its potent ACE2 inhibition. The study aims to assess the intervention effect on COVID-19 markers of the RAAS, such as angiotensin-II and angiotensin-(1-7) [
71]. Nutritional elements may support ACE inhibition indirectly by intercepting viral entry or via regulation and improvements in biomarkers associated with the involvement of the various systems [
71]. For example, excessive sodium ions can impair the endothelial vasculature and risk hypertension, but manifestations may be ameliorated with higher potassium ion levels. Hence, mushrooms, which generally contain high potassium and low sodium may be a good nutritional source for ACE inhibition as well [
1,
24].