Abstract
Obesity is associated with low-grade chronic inflammation and intestinal dysbiosis. Ganoderma lucidum is a medicinal mushroom used in traditional Chinese medicine with putative anti-diabetic effects. Here, we show that a water extract of Ganoderma lucidum mycelium (WEGL) reduces body weight, inflammation and insulin resistance in mice fed a high-fat diet (HFD). Our data indicate that WEGL not only reverses HFD-induced gut dysbiosis—as indicated by the decreased Firmicutes-to-Bacteroidetes ratios and endotoxin-bearing Proteobacteria levels—but also maintains intestinal barrier integrity and reduces metabolic endotoxemia. The anti-obesity and microbiota-modulating effects are transmissible via horizontal faeces transfer from WEGL-treated mice to HFD-fed mice. We further show that high molecular weight polysaccharides (>300 kDa) isolated from the WEGL extract produce similar anti-obesity and microbiota-modulating effects. Our results indicate that G. lucidum and its high molecular weight polysaccharides may be used as prebiotic agents to prevent gut dysbiosis and obesity-related metabolic disorders in obese individuals.
Introduction
Traditional Chinese Medicine has a long history in Asian countries dating back several thousands of years. One class of traditional remedies commonly in use consists of medicinal mushrooms such as Ophiocordyceps sinensis, Antrodia cinnamomea and Agaricus blazei Murrill, which contain a wide range of immuno-modulatory and bioactive compounds. One of the most intriguing medicinal mushrooms is the Basidiomycete fungus Ganoderma lucidum, which has been used for centuries to promote health and longevity. Previous studies have shown that triterpenes and polysaccharides isolated from G. lucidum inhibit adipocyte differentiation and produce hypoglycaemia effects in diabetic mice. In addition, proteoglycans isolated from G. lucidum fruiting bodies induce antidiabetic, antihyperlipidemic and antioxidant activities. However, it remained unknown whether G. lucidum produces any effect on body weight and obesity-related disorders.
Obesity is defined as a disease condition associated with numerous health problems and a reduced life expectancy. Growing evidence indicates that obesity is closely linked with chronic, low-grade inflammation, which can lead to insulin resistance, type 2 diabetes, fatty liver disease, cardiovascular disease, obstructive sleep apnoea and cancer. The high prevalence of obesity is currently a major threat to public health, with ∼500 million obese people and 1.4 billion overweight individuals worldwide. Prevention of obesity thus represents a major challenge for modern societies.
A recent study indicates that changes in the composition of the gut microbiota are associated with the development of obesity and its associated metabolic disorders. The gut microbiota comprises trillions of bacteria that contribute to nutrient acquisition and energy regulation. An increased ratio of the major phyla Firmicutes/Bacteroidetes and changes in several bacterial species can promote the development of obesity in both dietary and genetic models of obesity in mice. Other studies in obese animals suggest that obesity-induced gut dysbiosis caused by either environmental or genetic factors impairs intestinal integrity. This process leads to the release of the endotoxin lipopolysaccaride (LPS) from intestinal Gram-negative bacteria into the bloodstream, in turn, leading to metabolic inflammation and insulin resistance in obese mice due to stimulation of Toll-like receptor 4 (TLR4)-mediated inflammation. Moreover, chronic injection of LPS in mice leads to mild obesity and insulin resistance, highlighting a possible role for microbiota-derived LPS in obesity-induced inflammation.
A number of treatments, including antibiotics and prebiotics, are being evaluated for the management of obesity and its related metabolic disorders. For example, antibiotic treatment alters the gut microbiota, reduces blood endotoxemia and improves glucose tolerance in mice lacking the leptin gene (ob/ob mice) or in mice fed with a HFD. In addition, prebiotics are non-digestible, fermentable carbohydrates and fibres, which reduce body weight and exert anti-inflammatory effects mainly by enhancing the growth of specific beneficial bacteria found in the gut. Prebiotics not only alter the intestinal microbiota but also improve intestinal tight junction integrity and decrease blood endotoxemia caused by LPS. Prebiotics may, therefore, protect animals against obesity-induced inflammation.
In the present study, we examined whether a water extract of G. lucidum mycelium (WEGL) can decrease obesity in HFD-fed mice. Our results indicate that WEGL reduces obesity and inflammation in the treated mice. These effects are transmissible to HFD-fed mice through horizontal faeces transplantation, indicating that the effects of WEGL involve the gut microbiota. Characterization of WEGL showed that polysaccharides of molecular weight >300 kDa exerted similar ameliorative effects as WEGL. These results implied that the high molecular weight polysaccharides may be the active components of WEGL. Our data thus demonstrate that WEGL represents a potential prebiotic agent that may be used for the treatment of obesity and its complications.
Results
WEGL prevents HFD-induced obesity in mice
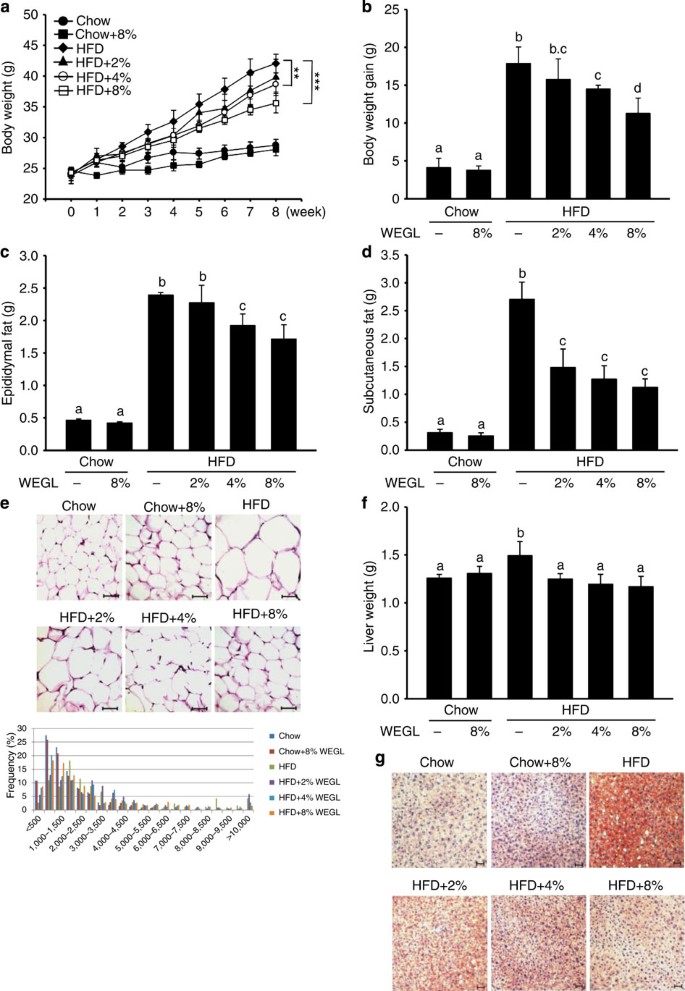
Using a mouse model of obesity, we observed that HFD feeding for 8 weeks led to significant increases in body and liver weight, epididymal and subcutaneous fat accumulation, and lipid deposition in adipocytes and hepatocytes compared with control chow feeding. While 8% WEGL did not produce any apparent effects in chow-fed mice, supplementation with WEGL decreased weight gain and fat accumulation in a dose-dependent manner in HFD-fed mice . Mean energy intake, stool fat and faeces energy did not vary significantly between HFD-fed groups (Supplementary Fig. 1), suggesting that the effects of WEGL on body weight and obesity parameters were not due to reduced food consumption or energy extraction. These results imply that WEGL reduces weight gain and fat accumulation in HFD-fed mice.
Chow- and HFD-fed mice were treated daily with 100 μl of either water or WEGL at 2, 4 or 8% (w/v) by intragastric gavage for two months (n=7 for each group). Effects of WEGL treatment on body weight (a) body weight gain (b) epididymal fat (c) subcutaneous fat (d) and epididymal adipocyte size (e) are shown. In e, adipocyte size was estimated using the Image J software (lower panel). Scale bar, 50 μm. Liver weight was measured in HFD and control, chow-fed mice (f). Liver lipid content was assessed using oil red O staining (g). Scale bar, 30 μm. Data are expressed as mean±s.e.m. Body weight differences in a were analysed using unpaired two-tailed Student’s t-test (**P<0.01, ***P<0.001). Graph bars in b, c, d and f marked with different letters on top represent statistically significant results (P<0.05) based on Newman–Keuls post hoc one-way ANOVA analysis, whereas bars labelled with the same letter correspond to results that show no statistically significant differences. In the case where two letters are present on top of the bar in b, each letter should be compared separately with the letters of other bars to determine whether the results show statistically significant differences.
WEGL reduces inflammation in HFD-fed mice
Previous studies have shown that HFD-fed obese mice produce higher levels of pro-inflammatory cytokines in hepatic and adipose tissues, including tumour necrosis factor-alpha (TNF-α), interleukin-1-beta (IL-1β), interleukin-6 (IL-6) and plasminogen activator inhibitor-1 . In contrast, production of the anti-inflammatory cytokine IL-10 is reduced in obese animals. We measured messenger RNA (mRNA) expression of these cytokines after 8 weeks of HFD feeding with or without WEGL supplementation. TNF-α, IL-1β, IL-6 and PAI-1 expression levels were higher in hepatic and adipose tissues of HFD-fed mice compared with tissues of control chow-fed mice, whereas IL-10 expression was reduced (Fig. 2a–e). Notably, the expression pattern of these cytokines was altered in a dose-dependent manner by WEGL treatment, resulting in expression levels closer to that of chow-fed mice than HFD-fed mice with increasing WEGL dose (Fig. 2a–e). Moreover, WEGL reduced the levels of secreted TNF-α, IL-1β and IL-6 proteins in a dose-dependent manner in the serum of HFD