1. Introduction
As defined by the World Health Organization (WHO), depression is a common mental illness that affects more than 322 million people worldwide [
1]. The typical symptoms of depression are persistent sadness, as well as the inability to feel feelings of happiness (anhedonia), sleep problems, loss of appetite, general fatigue, and cognitive problems. Depression is one of the leading causes of mental and physical disability worldwide [
2].
The etiology of depression is a complex interplay of psychological, social, and biological factors. People who have experienced adverse life events, such as the death of a loved one or prolonged unemployment, are at an increased risk of developing depression. The presence of physical diseases such as cardiovascular and neurogenerative diseases may increase the risk of depression [
3,
4].
Interestingly, the COVID-19 pandemic increased depressive symptoms by five percentage points, from 27.8 to 32.8% of adults in the United States in 2021 compared to the first months of 2020 [
5]. The increased risk of depressive symptoms and the development of depression may be related to the so-called pandemic environment and the introduction of “lockdowns” that restrict social activities in many countries. There is also growing evidence of COVID-19 disease and an increased risk of depression in recovered adults [
6]. The mechanism of the development of depressive symptoms in recovered adults is not well understood yet. One of the possible explanations for this phenomenon may be related to the so-called “cytokine storm”—abnormally high levels of pro-inflammatory cytokines such as IL–1β, IL–6, IL–12, and tumor necrosis factor-alpha (TNF–α) and interferon gamma (INF–γ). A cytokine storm can contribute to neurotoxicity, blood barrier disruption, or even acute necrotizing encephalopathy [
7].
Clinical psychopharmacology is a branch of science that deals with the description of the use of pharmacological agents for the treatment of specific psychopathological symptoms. The beginning of this field of knowledge dates to the 1940s and 1950s [
8]. The first antidepressant drug was iproniazid, introduced in the treatment of tuberculosis in 1952 [
9]. In tuberculosis patients treated with this drug, a significant improvement in mood was observed, unprecedented in patients in this clinical state [
10]. After a few years, the mechanism of action of iproniazid was described, as it turned out to be an irreversible inhibitor of the monoamine oxidase (MAO) enzyme, which in turn led to an increase in the concentration of biogenic amines in the brain [
11]. Iproniazid became a precursor drug for the first antidepressants, MAO inhibitors, including trancylopromine and phenelzine. Currently, iproniazide is not registered for the treatment of depression due to side effects, including liver damage [
11].
Treatment of depression is based on the theory of monoamines introduced in the 1960s, which states that this disease is caused by a decreased level of monoamines (serotonin, norepinephrine, and dopamine) in the brain [
12]. The mechanism of action of drugs used in the first line of depression treatment is inhibition of neuronal reuptake of monoamines from synaptic clefts, as in the case of selective serotonin reuptake inhibitors (SSRIs), for example, fluoxetine, citalopram, or sertraline, which reduce the activity of serotonin transporters [
13]. Although these drugs are potent antidepressants, the cause of depression is not simply insufficient monoamine levels. SSRIs cause an immediate increase in serotonin transmission, while it takes several weeks for mood-elevating activity to develop in treated patients, which is associated with changes in the expression of serotonin-dependent receptors. Recent data on the development of depression have extended the theory of monoamines to include neurotrophic and neurogenic hypotheses [
14,
15]. Decreased levels of brain-derived neurotrophic factor (BDNF) are involved in the pathogenesis of depression [
16]. BDNF is required for neurogenesis and neuroplasticity in the hippocampus [
17]. In people with depression, BDNF expression is decreased in the limbic area of the brain due to neuronal atrophy. Serotonin and its receptors are involved in the regulation of BDNF levels and neurogenesis in the adult hippocampus. Chronic treatment with an SSRI has been shown to increase BDNF levels in humans and rodents [
18,
19]. Altered levels of other neurotrophins, such as neurotrophin–3 (NT–3), neurotrophin–4 (NT–4), and nerve growth factor (NGF), are also observed in patients with depressive disorders [
20].
Medicinal/edible mushrooms and their mycelia from in vitro cultures are receiving increasing scientific attention for their potential to promote health. They are considered functional foods because of their ability to synthesize and accumulate different types of metabolites, which enhance their health-promoting properties and can be used as a supplement to the human diet. Studies show the multidirectional activity of medicinal mushrooms and their mycelium, including antioxidant, anticancer, anti-inflammatory, and immunostimulatory effects. Increasingly, there is also evidence of antidepressant activity [
21,
22,
23].
Researchers at Penn State University published a research paper describing the link between eating mushrooms and depression [
24]. The main conclusion of this population-based study, which analyzed mushroom consumption among US residents from 2005 to 2016, was that mushroom consumers are less likely to suffer from depression [
24]. The results are consistent with previous small clinical studies [
25,
26,
27]. However, the studies presented above did not investigate the potential mechanisms of the antidepressant effect of edible mushrooms.
This review aims to explain the antidepressant activity of edible/medicinal mushrooms by elucidating mechanisms from different perspectives, starting with answering the question of whether edible mushrooms can be a good source of indole compounds, such as L–tryptophan (Trp)—a precursor of the brain serotonin synthesis pathway. Although psilocybin-containing mushrooms are considered inedible, psilocybin and its active constituent, psilocin, is a real candidate for being classified as a rapid-acting antidepressant (RAAD) which is especially important for patients suffering from treatment-resistant depression (TRD). That is why this review also describes the current knowledge about the mechanism of action of psilocybin action and summarizes the current progress of clinical trials considering the usage of psilocybin in TRD. The review summarizes the anti-inflammatory effect of the administration of edible/medicinal mushrooms in alleviating neuroinflammation and the influence of analysis on the activity of the kynurenic pathway for in vitro and in vivo models. Furthermore, the neurotrophic and neurogenic activity of selected edible/medicinal mushrooms of in vitro and in vivo models was summarized. The last part of this review focuses on summarizing the current knowledge of edible/medicinal mushroom species—extracts or isolated substances on the gut microbiota, which has been extensively studied over the last five years.
2. L–Tryptophan Derivatives—Essential Compounds for Serotonin Synthesis
L–Tryptophan (Trp) and its derivatives, such as 5-hydroxy–L–tryptophan (5-OH-L-Trp), and tryptamine, are related to biochemical reactions that lead to serotonin synthesis in the brain’s neurotransmitters, lower levels of which are observed in clinically depressed patients [
28] These compounds have been shown to scavenge free radicals and protect cells against oxidative stress, potentially reducing the risk of certain diseases such as cancer, neurogenerative diseases, and depression [
29].
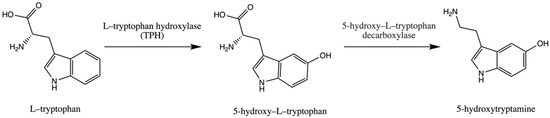
Trp is an essential amino acid and is considered an exogenic amino acid for the human body. Although its importance is the synthesis of various proteins, Trp is a precursor of serotonin (5-hydroxytryptamine) in the brain and gut. The biosynthetic pathway of serotonin is presented in .
Figure 1. Pathway of serotonin (5–hydroxytryptamine) synthesis.
The serotonin metabolic pathway starts with the hydroxylation of Trp to 5-OH-L-Trp, which is decarboxylated to 5-hydroxytryptamine (serotonin). The limiting stage of serotonin synthesis is Trp hydroxylation by the enzyme Trp hydroxylase (TPH) and is not saturated at physiological brain tryptophan concentrations; therefore, serotonin synthesis in the brain is assumed to be directly connected with tryptophan transport into the brain [
30,
31,
32].
Trp can be transported to the brain through a nutrient amino acid transporter protein that is involved in the transport of large neutral amino acids (LNAAs) such as valine, leucine, isoleucine, tyrosine, phenylalanine, and methionine from the bloodstream to the brain through the blood-brain barrier (BBB) [
33]. The content of Trp that crosses the BBB by the nutrient amino acid transporter depends on the ratio of Trp and other LNAAs in plasma [
33]. After meal ingestion, the levels of Trp and other LNAAs in plasma increase. As a result of a relatively low increase in Trp in comparison to other essential amino acids in plasma concentration, the plasma Trp/LNAA ratio decreases, and consequently, a reduced Trp influx to the brain is observed [
33].
There are several factors that can influence Trp influx to the brain by influencing LNAA concentration in plasma, such as the ingestion of carbohydrates, the intake of protein amounts, or exercise. Ingestion of dietary carbohydrates led to elevated insulin levels. Insulin promotes the uptake of LNAAs in skeletal muscle, which leads to an increase in the Trp/LNAA ratio and consequently to Trp influx into brain tissue [
34]. L–Tryptophan is not transported to muscle tissue because it bonds with albumin, while other LNAAs are not.
Trp obtained from food can be transformed into serotonin in a limited amount. In mammals, approximately 95% of Trp is metabolized through the kynurenic metabolic pathway, whose products exhibit biological activity [
35].
Fruiting bodies of edible mushrooms are a good source of non-hallucinogenic indole compounds such as Trp, 5-OH-L-Trp, and tryptamine () [
36,
37,
38].
Table 1. Content of L–tryptophan, 5–hydroxy–L–tryptophan and tryptamine in fruiting bodies of selected medicinal mushrooms.
The highest content of Trp and its hydroxylated derivative was observed in
Pleurotus djamor (respectively, 24.84 and 193.95 mg/100 g dw) and
Suillus bovinus (respectively, 25.9 and 15.83 mg/100 g dw) [
39].
According to scientific data, nonfungal sources that contain high levels of Trp include soy seeds (680 mg/100 g dw), pumpkin seeds (580 mg/100 g dw), and spirulina (930 mg/100 g dw) [
45]. Other researchers described that transgenic soybean plants were found to accumulate Trp at levels as high as 380 to 480 mg/100 g dw of seed flour, up to a 12-fold increase compared to Trp levels in non-transgenic seeds [
46]. Wheat—durum 169 mg/100 g dw, rye 125 mg/100 g dw, barley 165 mg/100 g dw, chickpea 220 mg/100 g dw, lentil—red 139 mg/100 g dw, and kidney beans 240 mg/100 g dw are also considered good natural sources of Trp [
45]. In contrast, the seeds of the
Griffonia simplicifolia plant are considered one of the best natural sources of 5-OH-L-Trp [
47,
48]. Its content can be as high as 156 mg/g dw (16% of the seed weight) [
48]. However, Maffei points out that mushrooms can also be a good source of this substance [
47]. When analyzing the plant sources of Trp, it turns out that tomatoes are a good source (14.71 mg/100 g dw), while smaller amounts were determined in strawberries (5.7 mg/100 g dw), lettuce (2.5 mg/100 g dw), spinach (0.65 mg/100 g dw) or chicory (0.08 mg/100 g dw) [
49]. Thus, it appears that both mycelium and fruiting bodies can provide an alternative source of Trp and Trp derivatives. The content of biologically active substances in mushroom samples is mainly measured after extracting them with various solvents such as methanol and ethanol from raw, lyophilized fruiting bodies. To determine the usage of selected edible mushrooms as sources of indole compounds, the influence of various types of thermal preparation of edible mushrooms on the content of biological active substances was analyzed [
40,
41]. Thermal processing (dry material suspended in water and thermostated at 100 °C for 60 min in a Soxhlet apparatus) was shown to result in approximately 2 times lower indole compound content after thermal processing compared to the unprocessed. However, Trp content increases relatively in processed samples compared to that of unprocessed one. The increase in Trp content can be explained by the fact that 5-OH-L-Trp or serotonin degradation at higher temperatures [
40]. The results were confirmed in another study [
41]. In conclusion, the method of preparing meals with mushrooms can affect the content of indole compounds because of their sensitivity to elevated temperature. However, thermally processed mushrooms remain a good source of Trp and 5-OH-L-Trp [
40,
41].
Today, dietary supplements containing lyophilized fruiting bodies, extracts, or even mycelium from edible mushroom species are available in community pharmacies or in stores with so-called healthy food. Mycelium can be obtained through in vitro cultures initiated from specially prepared parts of the fruiting body, the hymenial area. One of the most important advantages of in vitro cultures is the fact that the content of biological active substances does not differ between batches because the condition of the in vitro culture is monitored and maintained at specific parameters depending on the mushroom species. Studies have shown that the content of indole compounds in biomass from in vitro cultures can be much higher than that in fruiting bodies [
50]. The content of selected indole compounds in mycelia from in vitro cultures is presented in .
Table 2. Content of selected non-hallucinogenic indole compounds in mycelia of selected edible mushroom.
In most cases, the content of Trp is higher in mycelia than in the fruiting bodies of selected edible mushroom species, especially
Pleurotus citrinopileatus and
Pleurotus djamor. The most notable change can be observed in the content of 5-OH-L-Trp, which is almost four times higher in the mycelium compared to the fruiting bodies [
39]. Another advantage of making mycelium from edible mushroom species a dietary supplement is that powdered mycelium or mycelial extract does not have to be thermally processed, so thermolabile substances will not degrade.
Modification of the composition of the in vitro medium, such as the addition of indole precursors—anthranilic acid and serine—can have a positive influence on the content of indole compounds in mycelia [
55]. In one experimental study, in vitro culture medium was supplemented with various concentrations of serine or anthranilic acid (0.1–0.75 g/L). For in vitro cultures of
A. bisporus and
I. badia, the most optimal precursor concentration was 0.5 g/L of serine for
A. bisporus or 0.5 g/L of anthranilic acid for both species analyzed according to the content of indole compounds. The addition of 0.5 g/L of serine to
A. bisporus in vitro cultures resulted in the highest total concentration of indole compounds (186.37 mg/100 g dw). The addition of 0.5 g/L anthranilic acid to in vitro cultures of
I. badia and
A. bisporus resulted in the highest total concentration of indole compounds, 352.06 dw and >200 mg/100 g dw [
55].
The liberation of biological substances, which is the number of substances released from the matrix of food or dietary supplement formula (tablets, hard capsules), can be measured in vitro using models of the human gastrointestinal tract. Therefore, only substances free of their matrix can be absorbed in the gastrointestinal tract. The analysis of the liberation of indole compounds was performed for
Agaricus bisporus mycelia [
56]. In the study, the content of indole compounds in artificial gastric and intestinal juice was measured after 5 time points—15, 30, 60, 90, and 120 min of incubation. The highest 5-OH-L-Trp content was established between 91.99 and 324.64 mg/100 g dw after 30 min of digestion in artificial gastric juice and after 150 min of incubation in artificial intestinal juice [
56]. In a similar study on the release of indole compounds from fruiting bodies and
Tricholoma equestre mycelia, 5-OH-L-Trp was released in the highest amount from freeze-dried mycelia after 120 min of incubation in artificial gastric juice (352.47 mg/100 g dw) and after 15 min of incubation in artificial gastric juice in the case of fruiting bodies (281.56 mg/100 g dw) [
57]. For the fruiting bodies of
Suilius bovinus, the highest content of released 5-OH-L-Trp was observed after 120 min of incubation in artificial gastric juice (237 mg/100 g dw) (for liberation study of
Imleria badia,
Boletus edulis,
Cantharellus cibarius,
Lactarius deliciosus,
Leccinum scabrum,
Armillaria mellea,
Suillus luteus,
Pleurotus ostreatus,
Auricularia polytricha, see [
58]). Based on the studies mentioned above, it can be concluded that indole compounds are released in the highest amount in artificial gastric juice compared to artificial intestinal juice. Trp is not readily liberated, regardless of whether it is from fruiting bodies or mycelia from in vitro cultures. However, 5-OH-L-Trp was one of the indole compounds that was released at the highest amount, regardless of the species analyzed [
56,
57,
58].
Another important factor that should be considered when fruiting bodies or mycelium are thought to be a source of indole compounds is the bioavailability of these compounds. Bioavailability is a term used to describe the percentage or amount of a xenobiotic that reaches the systemic circulation [
59]. In the case of the bioavailability analysis of secondary metabolites such as indole compounds, it will be the amount of indole compound that reaches the systemic circulation. The evaluation of the bioavailability of natural compounds in humans is rare due to requirements and restrictions imposed by ethics commissions, therefore, alternative methods involving, for example, the colon epithelial cells (CaCo-2) cell line are used to estimate the bioavailability of active substances [
60]. In the study of indole absorption from
Imleria badia mycelia, the CaCo-2 cell line was used to measure active transport, while semi-permeable membranes were used in the passive transport model after release of biological active substances in the human gastrointestinal tract model. The bioavailability of 5–hydroxy–L–tryptophan ranged from 5.21 to 11.92% using active transport modes (depending on mycelial in vitro culture conditions—an addition of zinc (VI) sulfate or zinc hydrogen aspartate). Through the passive transport model, 5–hydroxy–L–tryptophan accounted for 2% of the compound released into artificial digestive juices [
61]
3. Tryptamine Derivatives—Psilocybin as a Potential Rapid Acting Antidepressant
Among patients with Major Depressive Disorder (MDD), almost 30% suffer from a treatment-resistant one [
62]. To date, there is no one definition of TRD, but the most common criteria found in the literature are: failure to respond to at least two antidepressants with different mechanisms of action treatment, confirmation of adequate dosage, and duration of treatment longer than 4 weeks for each antidepressant without effect [
63].
Patients suffering from TRD or for those for whom antidepressant action should be obtained in a shorter time compared to conventional — for example, patients at high risk of suicide may benefit from a novel group of antidepressants, rapid-acting antidepressants (RAADs) [
64].
The definition of RAADs has not yet been specified, but contrary to conventional antidepressants, which require a few weeks to produce significant antidepressant action, they need one or a few doses to produce a significant impact on depressive symptoms or even remission, especially in a group of patients who did not respond to first-line treatment [
65]. There are several mechanisms that can be responsible for the rapid antidepressant action of some drug candidates, for example, NMDA receptor antagonism, muscarinic receptors, and classic psychedelic drugs such as psilocybin or LSD, which influence serotonergic activity. The first drug registered for the TRD with a rapid-acting mechanism is esketamine, the ketamine enantiomer, approved by the US Food and Drug Administration and the European Medicines Agency in the form of a nasal spray called Spravato
® [
66]. Esketamine is a non-competitive NMDA receptor antagonist. It selectively induces an antagonist effect in the event of excessive activation of NMDA that leads to an increased concentration of extrasynaptic glutamate and activation of neuroplasticity pathways [
67]. The high effectiveness of esketamine in the treatment of TRD leads researchers to seek other candidates for RAADs such as psilocybin.
Psylocibin (3-[2-(dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate) is a natural substance which is a secondary metabolite found in the following genera:
Psilocybe, Copelandia,
Pluteus,
Gymnopilus,
Pholiotina,
Galerina,
Inocybe [
68,
69,
70,
71,
72,
73]. It is not biologically active and must be dephosphorylated to become psilocin, a psychoactive compound. Psilocin, as a classic psychedelic, has an agonist or partially agonist effect on 5-HT
2A receptors, which is a possible explanation of the hallucinogenic effect of this compound as these receptors are highly expressed in the visual cortex [
74]. The following theory was confirmed by the administration of a selective 5-HT
2A antagonist, ketanserin, to humans, which attenuated the hallucinatory effect of psilocybin [
75].
Administration of psilocybin induces down-regulation of 5-HT
2A receptors, that overexpression is observed in patients with major depression disorder [
76,
77]. Another theory postulates that downregulation of 5-HT
2A receptors may be influenced by increased synthesis of BDNF in the medial prefrontal cortex (mPFC) after administration of psilocybin. Elevated levels of BDNF can be explained by modulation of AMPA and NMDA receptors through the effect of psilocybin administration as an agonist to 5-HT
2A receptors [
78]. Activation of this receptor has a positive influence on cerebral neuroplasticity by increasing BDNF synthesis and increasing c-FOS factor expression in the anterior cingulate cortex and mPFC—areas of the brain implicated in depression [
79]. The secondary mechanism of antidepressant action is its anti-inflammatory effect of psilocybin by decreasing levels of TNF–α and IL–1β, which was demonstrated in the human U937 macrophage cell line by administering psilocybin-containing mushrooms’ water extract [
80]. An increase in the level of pro-inflammatory cytokines, such as TNF-α, is one of the causes of the activation of the kynurenic pathway in microglia and the production of neurotoxic compounds, hydroxykynurenine and quinolinic acid [
81].
The use of psychedelics in the treatment of MDD has gained scientific attention due to the relatively low therapeutic effectiveness of current psychopharmacological approaches and the increasing knowledge of the pathophysiology of depression. Recent meta-analysis of psychedelic therapy for depressive symptoms carried out by the Ko team revealed that the definitive clinical efficacy of the use of psychedelics such as psylocybin, LSD, or ayahuasca for depressive symptoms has not been demonstrated, partly due to the lack of a sufficient number of randomized clinical trials [
82]. The largest clinical trial on the use of psylocibin in treatment-resistant depression was carried out by the Goodwin team with participants from ten countries in Europe and North America. The trial consisted of 233 participants, divided into three groups that were given a single dose of 25, 10, and 1 mg (control) of psilocybin. The change in value of the Montogomery-Åsberg Depression Rating Scale, the tool that is used to stratify the severity of depressive episodes in adults, from baseline to week 3 was the primary endpoint of the trial. Psilocybin at a single dose of 25 mg reduced depression scores significantly more than a 1-mg dose over a period of 3 weeks, but was associated with adverse effects such as headache, nausea, and dizziness [
83]. Although there is no definitive verdict on the use of psychedelics in the treatment of Major Depressive Disorder, based on randomized clinical trials conducted with psilocybin, short- and long-term reductions in depressive symptoms have been observed [
83].
Gotvaldová and her team performed a quantitative analysis of tryptamine derivatives in fruiting bodies of genera such as
Psilocybe,
Pluteus, and
Inocybe [
84]. The highest content of psilocybin in the analyzed species of
Psilocybe genra was in
Pmexicana (3.29–3.93 mg/g dw),
P. caerulipes (2.23–5.67 mg/g dw),
P. cyanescens (2.34–13.8 mg/d gw), and
P. serbica var. moravica (5.65–14.16 mg/g dw). The highest concentration of psilocybin in
Pluteus genra was found in
P. americanus (1.17–2.43 mg/g dw). In
Inocybe genra, the amount of psilocybin did not exceed 0.282 mg/g dw (
I. corydalina) [
84]. Psilocybin is not present in edible mushrooms; concentration of this compound in
Agaricus bisporus was lower than the limit of detection [
84].
Few biotechnological attempts have been made to increase the content of tryptamine derivatives in fruiting bodies or mycelia in mushroom species containing psilocybin [
85,
86]. The most interesting biotechnological method for obtaining psilocybin is the production of this compound, which was proposed by the Milne team using metabolically engineered yeast (
Saccharomyces cerevisiae), whose productivity was determined at 120.3 mg/L of psilocybin. Due to further modification of the transformed metabolic pathway with
P. cubensis cytochrome P450 reductase, it was possible to obtain a production of psilocybin of 627 mg/L, which allows a relatively cheap production of psilocybin on an industrial scale [
85]. The highest production of psilocybin was observed in genetically modified
Escherichia coli in which 1.16 g/L was observed through the biotransformation of 4–hydroxindole, serine, and methionine. However, the method would be difficult to implement in an industrial setting because of the high price of substrates [
86].
Microdosing is the practice of repeatedly using low doses of psychedelics such as psilocybin. It is believed that the consumption of low doses of psilocybin (around 0.5 g per dose) can improve cognitive performance, stimulate creativity, and increase stamina. Microdoses do not induce hallucinations, contrary to the regular dose used for recreational use [
87]. Possession or consumption of psychedelics such as LSD or psilocybin is illegal in most countries, but there is increasing evidence of the use of microdoses of psychedelics. What is the main motivation for the consumption of “magic mushrooms”? In the survey that collected the responses from 1116 respondents through an online questionnaire, it was found that the main motivations for microdosing psychedelics were performance improvement (37%), mood improvement (29%), and curiosity (15%) [
88]. The consumption of microdoses of psychedelics is considered safe by those who decide to try it for the first time, but it showed that almost 20% of consumers experienced some acute psychological or physical negative effects [
88]. There are more studies describing microdosing phenomena among humans, but due to their type (online surveys, observation, and open-label studies). the results are prone to confirmatory bias, as many lack a control group or are based on self-selected samples [
88,
89,
90]. Some respondents reported a positive effect of microdosing on their cognitive abilities and creativity. However, it should be considered that in most cases, this may be due to the approach associated with the expectation of the positive effects of respondents and the researchers themselves conducting the observational study. A double-blind, placebo-controlled study of psylocybin microdosing carried out with 35 participants revealed no evidence to support enhanced cognitive or creative function. Low doses of psilocybin (0.5 g of dried fruiting bodies of
P. cubensis two times a week, the total dose equals to 1.0 g) even resulted in small cognitive impairments [
91].
So far, the results of clinical trials on the use of psilocybin in controlled clinical conditions give hope to patients suffering from TRD. In contrast to the antidepressants currently used, their effect appears several hours after the first dose of the preparation and lasts several days, which means that the total number of doses is lower than in the case of drugs administered every day, which can positively affect adherence to medical recommendations. More clinical trials are needed before psilocybin can be approved for the treatment of patients with TRD, especially those evaluating long-term antidepressant effects.
4. Anti-Inflammatory Activity of Medicinal Mushrooms in Beating Depression
The link between immune system, inflammation and depression was observed for the first time when IFN–α therapies, which activate inflammatory antiviral response, were introduced as a treatment for hepatitis C. Patients treated with interferon developed depression-like behaviors after 4 weeks of treatment initiationinitiation [
92]. Patients with MDD have been observed to have higher levels of pro-inflammatory mediators such as IL–6, IL–12 and C–reactive protein compared to nondepressed individuals [
93,
94]. Patients with TRD are more likely to have elevated pro-inflammatory markers [
95].
Peripheral inflammation can affect the central nervous system in many ways. It starts by having a negative impact on the permeability of the blood-brain barrier, which makes cytokines and immune cells more likely to cross to the brain [
96]. The possible crossing of the proinflammatory cytokines to the brain may alter the kynurenic pathways, which are correlated with tryptophan availability.
The activity of enzymes involved in the kynurenic pathway can be modulated by glucocorticosteroids and/or pro-inflammatory cytokines. The hypothesis of depression induction caused by tryptophan depletion in the brain was first stated by Fuch et al. in 2002 [
97]. However, research carried out by Dunn and Welch demonstrated that administration of LPS and/or proinflammatory cytokine IL–1 to mice increases brain tryptophan and serotonin concentration [
98]. The O’Connor team conducted a similar observation that showed that LPS administered to rodents resulted in increased kynurenine content in the brains of mice and brain tryptophan and serotonin [
99]. In human studies, patients treated with IFN–α showed that tryptophan concentrations in cerebrospinal fluid were stable despite a decrease in Trp blood level [
100].
Based on the observations mentioned above, it is unlikely that depressive symptoms can be caused or worsened by tryptophan depletion in the brain by shifting tryptophan to the kynurenic pathway. An alternate hypothesis is that alterations in concentrations of products of the kynurenine pathway may play a role in the development of depression [
81,
101].
Kynurenine can be metabolized in several ways, depending on the cell type in which kynurenine is produced, transported, and metabolized. In microglia, kynurenine is broken down to 3–hydroxykynurenine and quinolinic acid, which are neurotoxic. Neurotoxicity of these compounds is caused by the generation of reactive oxygen species that may damage neural cells and act as agonists in the NMDA receptor [
102]. Reactive oxygen species can promote the production of proinflammatory cytokines via NF-κB pathway [
103]. In astrocytes, kynurenine is degraded to kynurenic acid knows from its neuroprotective activity by acting as an antagonist of the NMDA and alpha-7 nicotinic acetylochine receptor [
104]. The intact neuron can metabolize kynurenine to picolinic acid, which is also neuroprotective.
In patients with depression, variations in the levels of the products of the kynurenic pathway have been observed. In a meta-analysis carried out by Ogyu et al. it was observed that in depressed patients decreased level of kynurenine and kynurenic acid was observed whereas depression free patients were observed with higher level of quinolic acid [
105].
Neuroinflammation may be beneficial because activation of microglia is necessary to eliminate the threat in the form of infection, injury, or toxic metabolites [
102]. Although chronic neuroinflammation can lead to overproduction of pro-inflammatory cytokines and production of neurotoxins (products of the kynurenic pathway described above) that can lead to neuronal death and, consequently, loss of neuronal volume in areas responsible for mood regulation such as the PFC or the hippocampus. Additionally, neuroinflammation may be an important part of the pattern of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [
106]. In vitro and in vivo studies showing the anti-inflammatory activity are presented in .
Table 3. Anti-inflammatory activity of selected medicinal/edible mushroom species.
Research on potential anti-neuroinflammatory activity is based on activity analysis of TLR4 and NF-κB pathways in cells in order to observe whether the addition of investigated mushroom species extract/isolated substance exhibits this activity.
Toll-like receptors (TLRs) are a group of transmembrane receptors responsible for the recognition of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). The activation of TLR4 receptor by bacterial lipopolysaccharide (LPS), viral proteins and polysaccharides results in the production of inflammatory substances, which are essential in order to produce effective immune response [
123]. Activation of TLR4 leads to activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). NF-κB is one of the most important and versatile family of transcriptional factors which are associated with inflammation and immunity [
124]. After stimulation, NF-κB factors complex are being freed and in free form (p50 and p65) translocate to nucleus which activate transcription and liberation of pro-inflammatory mediator, such as inducible nitric oxide synthase (iNOS) and NO, prostaglandin E2 (PGE2) and cyclooxygenase–2 (COX-2) and proinflammatory cytokines such as IFN-γ, IL-1β, IL-6, and TNF-α [
124].